17 de diciembre 2020
Abstract:
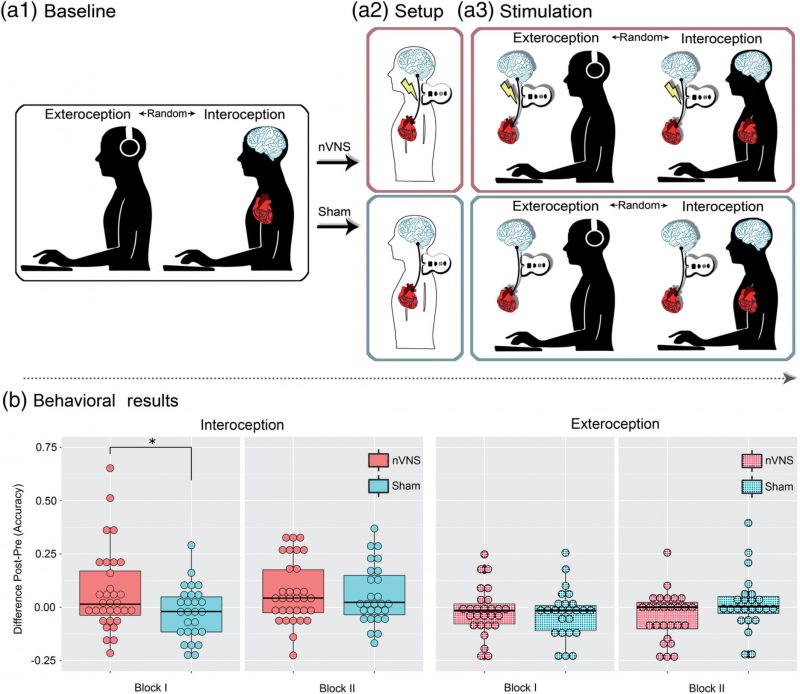
An accruing body of research has shown that interoception (the sensing of signals from the body’s internal milieu) relies on both a direct route (afforded by the vagus nerve) and a secondary route (supported by somatosensory mechanisms). However, no study has causally tested the differential role of these pathways, let alone via direct stimulation. To bridge this gap, we tested whether multidimensional signatures of interoception are modulated by noninvasive vagus nerve stimulation (nVNS). Sixty‐three participants were divided into an nVNS and a sham‐stimulation group. Before and after stimulation, both groups performed a validated heartbeat detection (HBD) task including a genuinely interoceptive condition (monitoring one’s own heartbeat) and a control exteroceptive condition (tracking an aurally presented heartbeat). Electroencephalographic signals were obtained during both conditions to examine modulations of the heartbeat‐evoked potential (HEP). Moreover, before and after stimulation, participants were asked to complete a somatosensory heartbeat localization task. Results from the interoceptive condition revealed that, after treatment, only the nVNS group exhibited improved performance and greater HEP modulations. No behavioral differences were found for the exteroceptive control condition, which was nonetheless associated with significant HEP modulations. Finally, no between‐group differences were observed regarding the localization of the heartbeat sensations or relevant cardiodynamic variables (heart rate and or heart rate variability). Taken together, these results constitute unprecedented evidence that the vagus nerve plays a direct role in neurovisceral integration during interoception. This finding can constrain mechanistic models of the domain while informing a promising transdiagnostic agenda for interoceptive impairments across neuropsychiatric conditions.